
[팟캐스트] 기존 Cell Line Development의 도전과제와 Monoclonality 조절에 도움이 되는 신기술
유전 공학 및 합성 생물학의 발전은 최근 수십 년 동안 수많은 혁신적 발견이 가능하도록 했습니다. The importance of cell line development needs an honorable mention. Without it, many life-saving vaccines for infectious diseases, antibody drugs, and recombinant protein products, including insulin would not be widely available The most challenging step of compliant cell line development is monoclonality assurance, primarily when carried out manually. Monoclonality of therapeutic cell lines must be accomplished and documented for regulatory reasons.
In our podcast, “Cell line development with Dr. Natasa Skoko, ICGEB & Dr. Hugh Graham, MacroGenics,” we discuss traditional workflows for cell line development and the emerging technologies to help verify monoclonality.
Here we’ve summarized key discussion points from expert speakers: Dr. Natasa Skoko of Group Leader of the Biotechnology Development Unit at the International Centre for Genetic Engineering and Biotechnology (ICGEB), and Dr. Hugh Graham, Director of Cell Culture Sciences at MacroGenics.
Cell Line Development
Monoclonality Assurance
Emerging Technologies and Regulatory Impact
Significance of stable cell lines
In the simplest form, the term cell line defines a population of cells that can be maintained in a culture for a certain period and retain a distinct phenotype, function, and stability.
Dr. Skoko summarizes the historical significance of the stable cell line. “The use of the cell line made the revolution, not just in scientific research and helped in understanding many fundamental biological processes,” Its applications range from antibody and therapeutic protein production to drug screening.
Cell line development involves engineered mammalian cells that are robust, cost-effective, and easy to grow. One cell line that fits all the criteria is the Chinese Hamster Ovary (CHO) cell, first developed in 1987. Today, 70% of biotherapeutics on the market are manufactured from CHO cell lines. They are particularly efficient in the mass production of biotherapeutics, thanks to their adaptability to various growth media and growth conditions (e.g. suspension, attached growth, fed batch, perfusion) as well as their ability to fold and glycosylate proteins to mimic post-translational modifications in human proteins.
Challenges of a traditional cell line development workflow
The production of high levels of biotherapeutics relies on the generation of stable cell lines.
The first step is transfection of a suitable host cell line with the gene of interest, leading to random integration of DNA into the host genome. Once the engineered cell line starts growing, usually under selection pressure, single-cell isolation is needed to generate clones for screening.
In traditional workflows, cells are plated onto a 96-well plate through the limiting dilution. This method involves the dilution of the cell suspension such that when you plate a certain amount into a well, the likelihood of it having only one cell is very high. Some wells can even be empty and a few may have multiple cells. Therefore, you have a high probability that a cell line from any one of these wells is developed from a single cell.
This is great, but how long does it take? Assuming cells double in numbers every 24 hours, it takes ten doublings to reach a thousand cells, which would take 10 days. To obtain enough cells to work with, would take 2-3 weeks. Moreover, regulations for monoclonality require that you repeat limiting dilution to have further assurance of clonal origin for your cell line. So, you would need to spend months to obtain results from this low-throughput method. Dr. Graham adds: “The stability of the cell line is challenged. By the time you reach the desirable numbers and have high assurance of monoclonality, there is a chance they will have drifted in their properties, lost some copies of the gene of interest, or adapted in a way you didn’t expect.”
To sum up, limiting dilution can fail to achieve speed, compliance and cell line quality because it is hard to document monoclonality and retain the quality of your cells. Both Dr. Skoko and Dr. Graham agree that automation is the way to facilitate high throughput monoclonal cell line growth and its documentation.
Essentials of monoclonal cell line
Let’s now take a step back to define monoclonality. A monoclonal cell line originates from a single cell or from a single progenitor.
Why is it such a big deal? As cells start to grow and double, they are subject to genetic drifts, mutations, or loss of a plasmid. So, it is nearly impossible to ensure that the quality of the therapeutic proteins they express is uniform by simply investigating their most recent phenotype.
To compensate for that, you need to start documenting your cells on Day Zero to prove that they indeed came from a single cell.
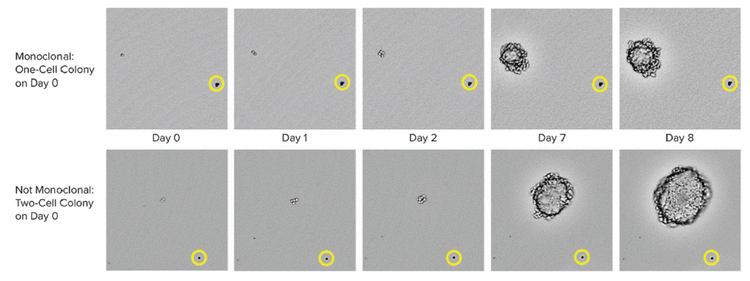
CHO-s cell growth was used to capture images from the 6-well plates at multiple time points. 0일 차에, 위 열에 하나의 세포가 존재한다는 것이 명확하게 관찰되었고, 아래 열에 두 개의 세포가 관찰되었습니다. The yellow circle shows the position of a bead that serves as a location reference to confirm that the same colony is imaged over time.
Typical monoclonality evidence that regulators look for is image-based, i.e., the image of a single cell must be recorded.
The most effective monoclonal cell line development equipment is a single cell printer, which performs single-cell deposition. Other methods include Fluorescence-Activated Cell Sorting (FACS) and workflows that will automate every step, from incubation to pipetting.
Importance of verifying early monoclonality
Monoclonality assurance at an early stage has several implications for life science applications. Dr. Graham highlights the significance of monoclonality for the drug development cycle: “There are cell lines and molecules going towards commercialization that were developed ten or more years ago. The cloning processes from ten years ago may not have the documentation required to assure a clonal origin. When one takes such a cell line to a regulator, you might be asked to show that it originally came from a clonal cell line. If you don’t have that proof, then proving it later in the project is a lot of work.”
Dr. Skoko adds: “It’s all about minimizing risk and having a consistent production process and product quality. That’s why it’s essential to assure monoclonality at the early stage. Starting with a cell bank with a high assurance of monoclonality results in less work later. Furthermore, you will avoid serious disruptions in manufacturing and reduce uncertainty when making changes to the manufacturing process.”
Emerging monoclonal technologies to verify single cell lines
Emerging techniques for monoclonal cell line imaging and cultivation are growing. Here are some examples:
- Semi-solid cell suspension, whereby a single cell grows in a semi-solid suspension, can be imaged as it grows.
- A cloning ring surrounding a single cell to isolate it and protect it from contamination
- FACS: The sorting of cells based on fluorescent characteristics
- Microfluidic drop-based single-cell printers with the ability to image newly-plated wells containing single cells
These methods combined improve the credibility of monoclonality evidence.
Of course, there is great potential to improve the current techniques and invent superior ones. First and foremost, visual evidence, i.e., single-cell imaging, must be used more often since it overcomes the cost and time-related challenges of limiting dilution and flow cytometry sorting.
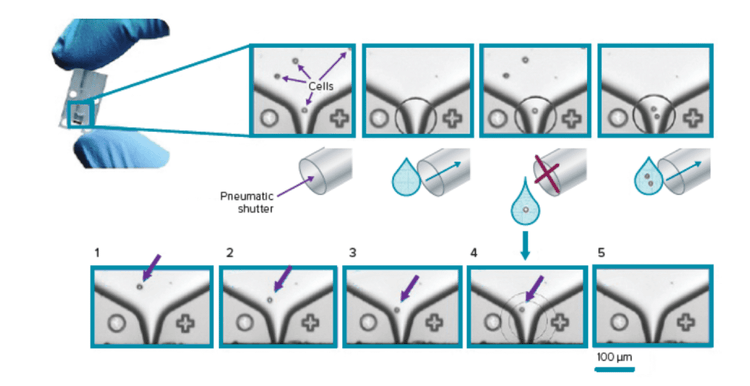
CloneSelect 단일 세포 프린터 시리즈는 완전 자동화 시스템으로, 단일 세포를 표준 Microplate에 정렬 및 배치하기 위해 독점 미세 유체공학 기반 기술과 실시간 이미지 분석을 활용하는 동시에 이미지 문서화로 Monoclonality를 보장합니다.
Further improvements are possible down the production line. For instance, improving analytical methods in therapeutic protein production can help us monitor the entire manufacturing process. This brings genetic variation under control and ensures uniformity of the manufactured protein.
Another concern in monoclonal cell line development is speed. As Dr. Graham points out, “Waiting for a clonal cell line to make your protein to go into a clinic is a barrier in terms of time. Today, people are moving towards the first batches of proteins from pools of early clones for the first Good Manufacturing Practice (GMP) clinical material. Then, one generates the clonal cell line for later-stage clinical trials and GMP production. Eliminating the need for proof of clonal origin in early clinical trials is an interesting approach to accelerate biotherapeutics development. However, this approach may need additional work to gain regulatory approval.”
The role of regulation and its impact on the cell line development process
As you can see, regulations undeniably impact monoclonal cell line development. One implication of stringent regulations is that the manufacturer must provide a comprehensive report on the monoclonal cell line before requesting approval. Insufficient proof or missing documents may result in regulatory delays or additional post-approval commitments. Because regulatory approval requires excellent scientific support, it is best to provide as much proof from the early stages as possible, especially for the clonal origin.
What does the future hold for monoclonal cell line workflows?
There are unresolved challenges in monoclonal cell line workflows, including financial concerns, obtaining high-throughput results, and assurance of monoclonal origin.
Compared with legacy methods, today’s monoclonal cell line developers have much better tools to ensure regulatory compliance and cell line quality.
One exciting prospect is improved data management, which is necessary to organize large datasets generated from cell line development studies. Combining data management with machine learning can give rise to a platform that predicts the quality of a biotherapeutic based solely on clonal origin.
The role of automation will also become more pivotal in speed and consistency. More specifically, automation can accelerate clone development and colony screening. Dr. Skoko clarifies the effects of automation: “We are talking about screening 10,000 clones in a couple of weeks. A traditional workflow would take anywhere from 30 weeks to two years. Automated technology can help us have accurate clones, eliminate errors associated with the traditional limiting dilution, and overcome working with a heterogeneous population of different secretors. You can evaluate the stability of your clones from the very beginning.”
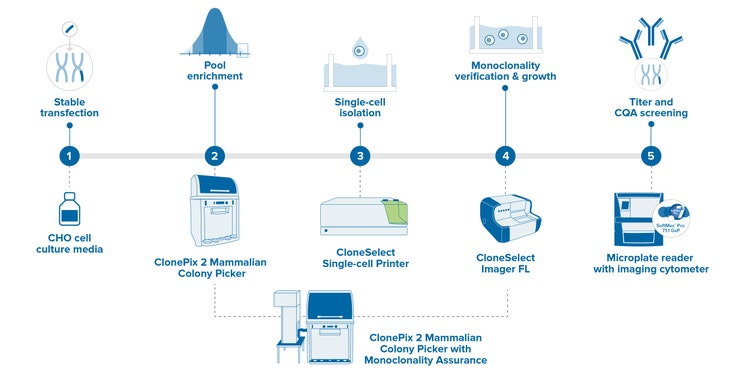
Automated cell line development workflow with monoclonality assurance
With the integration of automation technologies, you can screen hundreds of thousands of clones to find that golden clone that can generate the highest yield for the protein of your interest. More importantly, it helps you fully document every step of the development process from day zero. Ultimately, you get to save money and time by avoiding future conflicts caused by a lack of monoclonality assurance or inconsistent product quality.
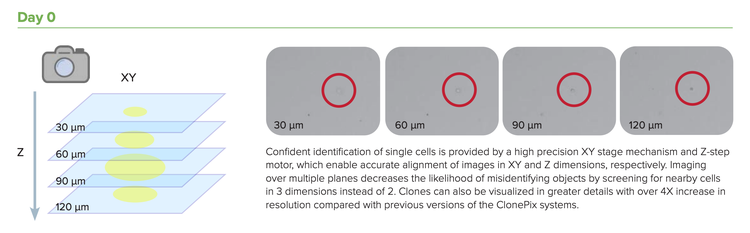
Accelerate your cell line development timelines by integrating multiple steps into a single step. The enhanced ClonePix 2 Mammalian Colony Picker with monoclonality assurance can automatically screen and pick clones that are both high producing and monoclonal—all in one system. 0일차에 더 짧은 단일클론 검증 시간 동안 더 많은 클론을 스크리닝하고, 2주 이내에 가장 생산량이 많은 클론을 스크리닝하여 피킹합니다. Rapid Z-stack acquisition feature allows detection of single cells throughout the medium volume, not just a single focal plane.
Molecular Devices has a robust portfolio to automate your cell line development workflow and technologies to assure monoclonality, including clone picking, single-cell isolation and imaging, and microplate readers. Our proven GxP solutions help assure data integrity and compliance for GMP/GLP labs.
Learn more about cell line development and monoclonality applications or speak to an automation specialist if you’re interested in exploring an automated solution for your lab.