Cell Line Development에서 CHO 세포 역할의 진화
Since the first approval of recombinant insulin and human growth hormone in the early 1980s, a multitude of recombinant protein therapeutics have been approved by regulatory agencies, notably the FDA in the US and EMA in Europe. Given this significant increase in successful introduction of biological therapeutic agents, there is a crucial need in the drug discovery space to support more efficient manufacturing processes that require highly productive cell lines.
The Chinese Hamster Ovary (CHO) cells are an epithelial-like cell line that are highly amenable to transfection and have emerged as the gold standard for the manufacturing of approved therapeutic proteins.
Why are CHO cells used?
Several key properties of CHO cells have driven their establishment as the preferred host cell line for regulatory approvals of recombinant therapeutic products:
1
Adaptable to growing in suspension culture, which is ideal for large scale production in bioreactors
2
Adaptable to growth in serum-free and chemically defined (animal-free) media supplements, which ensures reproducibility between different batches of cell culture
3
Allow posttranslational modifications (e.g. glycosylations) to recombinant proteins which are compatible and bioactive in humans
4
Several chemical selection and gene amplification systems have been developed for CHO cells, optimized for higher yield of recombinant protein per cell.
History of CHO Cells
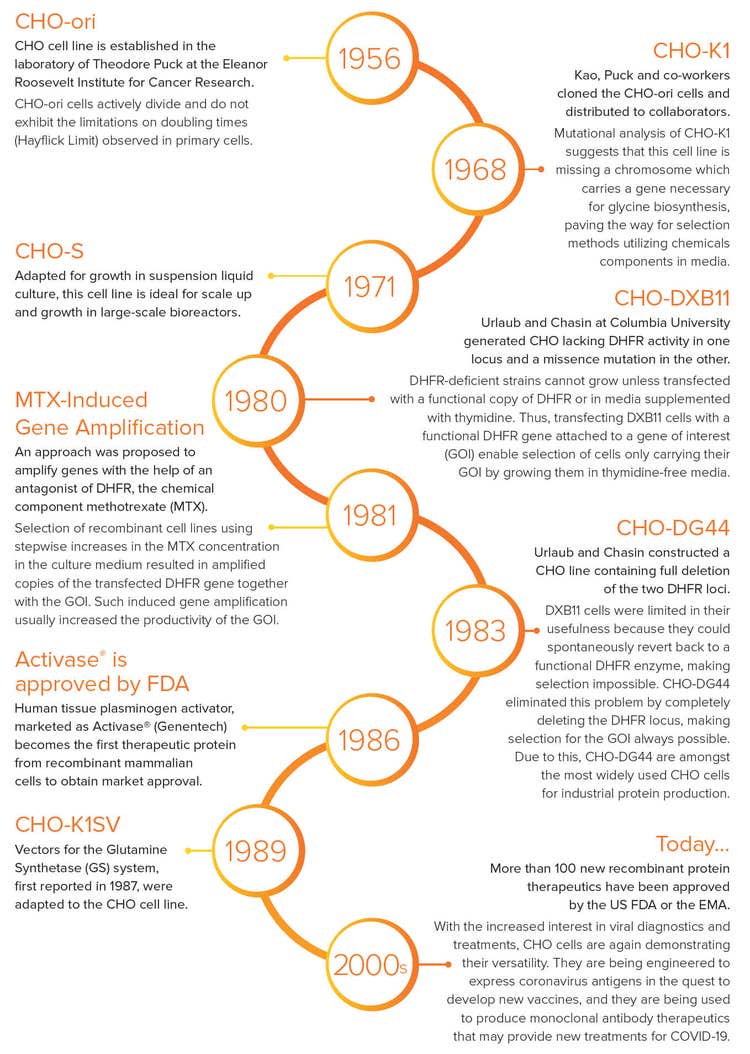
1956 - CHO-ori cells
CHO cell line is established in the laboratory of Theodore Puck at the Eleanor Roosevelt Institute for Cancer Research. CHO-ori cells actively divide and do not exhibit the limitations on doubling times (Hayflick Limit) observed in primary cells.
1968 - CHO-K1 cells
Kao, Puck and co-workers cloned the CHO-ori cells and distributed to collaborators. Mutational analysis of CHO-K1 suggests that this cell line is missing a chromosome which carries a gene necessary for glycine biosynthesis, paving the way for selection methods utilizing chemicals CHO-S components in media.
1971 - CHO-S cells
Adapted for growth in suspension liquid culture, this cell line is ideal for scale up and growth in large-scale bioreactors.
1980 - CHO-DXB11 cells
Urlaub and Chasin at Columbia University generated CHO lacking DHFR activity in one locus and a missence mutation in the other. DHFR-deficient strains cannot grow unless transfected with a functional copy of DHFR or in media supplemented with thymidine. Thus, transfecting DXB11 cells with a functional DHFR gene attached to a gene of interest (GOI) enable selection of cells only carrying their GOI by growing them in thymidine-free media.
1981 - MTX-Induced Gene Amplification
An approach was proposed to amplify genes with the help of an antagonist of DHFR, the chemical component methotrexate (MTX). Selection of recombinant cell lines using stepwise increases in the MTX concentration in the culture medium resulted in amplified copies of the transfected DHFR gene together with the GOI. Such induced gene amplification usually increased the productivity of the GOI.
1983 - CHO-DG44 cells
Urlaub and Chasin constructed a CHO line containing full deletion of the two DHFR loci. DXB11 cells were limited in their usefulness because they could spontaneously revert back to a functional DHFR enzyme, making selection impossible. CHO-DG44 eliminated this problem by completely deleting the DHFR locus, making selection for the GOI always possible. Due to this, CHO-DG44 are amongst the most widely used CHO cells for industrial protein production.
1986 - Activase® is approved by FDA
Human tissue plasminogen activator, marketed as Activase® (Genentech) becomes the first therapeutic protein from recombinant mammalian cells to obtain market approval.
1989 - CHO-K1SV cells
Vectors for the Glutamine Synthetase (GS) system, first reported in 1987, were adapted to the CHO cell line.
2000 - Today…
More than 100 new recombinant protein therapeutics have been approved by the US FDA or the EMA.
With the increased interest in viral diagnostics and treatments, CHO cells are again demonstrating their versatility. They are being engineered to express coronavirus antigens in the quest to develop new vaccines, and they are being used to produce monoclonal antibody therapeutics that may provide new treatments for COVID-19.
Generate recombinant CHO cells
Discover an effective and rapid process for generating recombinant CHO cell lines
The selection of high-producing mammalian CHO cell lines continues to represent a major bottleneck in process development for the production of biopharmaceuticals. Therefore, it is increasingly important to develop new high-throughput methods for the selection of high-expressing CHO cell lines in an efficient and cost-effective manner.
In this application note, discover an effective and rapid process for generating recombinant CHO cell lines, producing high levels of therapeutic proteins.

Molecular Devices provides a fast, simple and comprehensive solution for CHO cell line development. When used together CHO Growth A, CloneSelect Imager, and ClonePix 2 System enable researchers to more efficiently develop new protein-producing CHO cells lines, thus accelerating time to market.