Advantages of ELISA for Accurate and Accelerated Drug Discovery [Podcast]
Since its discovery nearly 50 years ago, the Enzyme-Linked Immunosorbent Assay (ELISA) has become the gold standard in diagnostics and drug evaluation studies. Using an ELISA, one can quantify a molecule of interest (e.g., protein, peptide, or hormone) in a liquid sample by immobilizing it on a microplate, using specific antibodies to bind that molecule, and detecting the particular binding with high sensitivity. The technique has remained popular, despite the emergence of other methods like flow cytometry, due to its specificity and versatility.
Uncover the detailed discussion of how our experts use ELISA assays in their research, the specific benefits of this kind of immunoassay, and what research directions have been opened up through the use of ELISA, and much more in our recent podcast:
Three accomplished scientists discuss what makes the ELISA stand out and how it can be adapted to the rapidly evolving drug discovery scene. Key learning points:



ELISA applications in drug discovery and development
Our three experts discuss how they use ELISA assays to support their research in drug discovery and development.
Efficacy of therapeutic antibodies against cytokines
For Kyowa Kirin, the ELISA is a critical checkpoint to assess the efficacy of therapeutic antibodies against inflammatory cytokines. By measuring the levels of cytokines, such as interferon-gamma, IL-8, and TNF-alpha, Dey and her colleagues assess the ability of their antibody clones to block the production of these cytokines. This helps them select the most effective antibody clones.
Assess the pharmacokinetic properties of therapeutic antibodies
ELISAs have also helped Dr. Miletic and Ms. Dey assess the pharmacokinetic properties of therapeutic antibodies. In these studies, the team measures the presence of the antibody in a patient's blood at various time points. This process helps them determine the half-life of the therapeutic, information that guides their selection of the most stable antibody clones.
Assess the reduced levels of p53 in knockout cells
Molecular Devices has demonstrated the value of a variety of ELISAs for a wide range of research approaches, including CRISPR knock-out experiments. One example is the knock-out of the p53 gene, which encodes a tumor suppressor. Using an ELISA to assess the reduced levels of p53 in knockout cells demonstrates the usefulness of the method for gaining insights into gene function, which could ultimately drive the development of novel targeted therapies.
Advantages of ELISA assays compared to other immunoassays
The three examples above demonstrate the versatility of ELISAs, but additional factors distinguish them from other immunoassays.
Cathy Olsen emphasizes the flexibility and sensitivity of ELISAs: “ELISAs can be very highly sensitive and specific. They are typically available with different readouts, depending on what is needed. Colorimetric ELISAs that use absorbance detection as a readout has been around for 30 years. Still, there are also fluorescent and luminescent readouts that can give you better sensitivity and better dynamic range. Thanks to high sensitivity, an ELISA can detect low-abundance molecules in the sample.”
For Ana Miletic, specificity is a critical attribute of ELISAs, which allows them to distinguish between closely related proteins. That said, the choice of antibody is the key. “An ELISA is only as good as the antibodies used in it. The backbone of any ELISA assay, which makes it a great assay, is the use of particular, high-affinity antibodies to your target of interest.” In other words, the correct antibody choice helps ensure the target analyte is detected and minimizes the risks of cross-reactivity with similar molecules.
Another advantage of the ELISA is its simplicity, with only plates, pipettes to add samples, and plate readers needed (a microplate washer can be added to ease the workload). Despite their undemanding design, ELISAs can provide a wide range of readouts. More importantly, since most of the equipment is automation-friendly, one can make ELISAs even more accessible and streamlined.
ELISA instrumentation and automation workflow solutions
An automated ELISA workflow involves several upgrades to the manual version, but the extent of automation ultimately depends on throughput needs.
Automated liquid handlers are very useful for sample preparation and reagent addition, while robotic arms can handle the transfer of plates between different sections of the system. Combining these two elements means one does not have to manually pipette reagents or carry the plates from the bench to the liquid handling system, back, and to the plate washer and reader. Since the liquid handler and robotic arm handle these steps, the researcher earns more time to run more focus-intensive experiments.
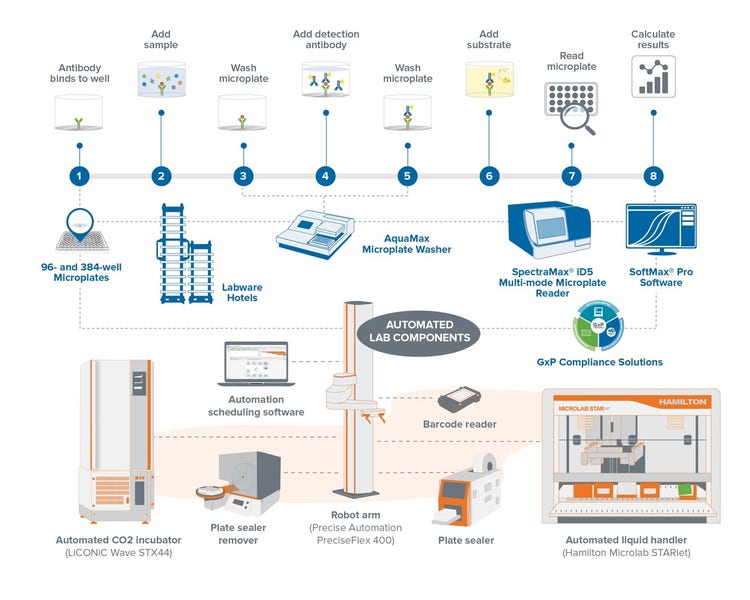
Two additional components make the workflow even more streamlined. One of them is the automation scheduling software that coordinates the workflow timeline and sequence to get more timely, accurate results. Cell-based assays require a stable environment for cells (e.g., 37oC, 5% CO2), and an automated incubator is used to maintain these conditions consistently.
Nevertheless, Cathy Olsen considers the plate reader a key to success in automated ELISA. “The quality of results depends very heavily on accurately detecting the signal in the ELISA plate. When you analyze your data, you want to get accurate results and quantitation of that target protein, which falls on the plate reader.” A plate reader’s software may offer automated analysis using protocols configured for a particular assay.
The automated ELISA workflow has two positive outcomes. First, as mentioned before, the walkaway time significantly increases, allowing researchers to multitask. More importantly, it can reduce human error with uniform sample preparation and a well-monitored liquid handling procedure.
What the Future Holds for ELISA
Many scientists continue to harness the qualitative and quantitative powers of ELISAs, and a current market analysis report predicts that the global research ELISA market will rise from US$519.4 million in 2022 to US$754.38 million by 2030.
ELISAs continue to serve as the benchmark for diagnosis by detecting specific antigens in bodily fluids, and healthcare professionals use them in various fields, including HIV testing, pregnancy tests, and the detection of infectious diseases.
In the recent COVID-19 pandemic, ELISAs have played a significant role in expanding our understanding of the disease mechanisms involved by enabling the detection of antibodies against the spike protein, nucleocapsid antigen, and other viral proteins. By measuring antibodies produced in response to vaccination, researchers could also assess the potential efficacy of a vaccine and optimize its formulation.
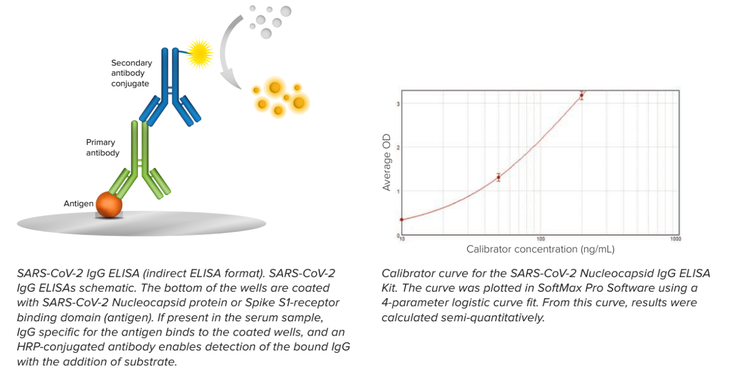
ELISAs can also help to elucidate the working mechanism of a drug. Even in the presence of a target protein, one needs to investigate how targeting a specific protein works. By introducing a drug molecule to different cell types and quantifying the levels of target proteins in these cells, researchers can deduce the impact of the drug on various cellular processes.
Future work must focus on addressing some of the limitations of ELISA to expand its potential. One key area is increasing sensitivity to accurately detect low levels of antigens in a sample, which can be achieved through signal amplification techniques. Additionally, optimization of blocking agents can reduce background noise and prevent non-specific antibody binding or cross-reactivity with similar antigens. Lastly, the addition of multiplexing is an upgrade enabling the quantification of multiple proteins, peptides, and small molecules in the sample simultaneously.
Discuss your automated ELISA solution
Automation does not have to be overwhelming – there are a lot of flexibility and options available. Our five ready-made workcells for ELISA workflows offer automation solutions ranging from simple plate-loading capabilities to more advanced, completely automated workcells and the best part, it can be customized and built over time. Automating labor-intensive plate-based assays increases walkaway time, throughput, and reproducibility by reducing a researcher’s need to engage in common, repetitive, hands-on tasks.
https://share.vidyard.com/watch/Nyr9umn2JZ2JaeMkQGw3jQ
Learn more about our ELISA workcells or when you're ready to speak to a workflow automation specialist about your application, contact us.