COVID-19에 대한 단클론항체의 역할
mAbs가 SARS-CoV-2와의 싸움에서 핵심인 이유를 살펴보고 어떻게 팬데믹이 mAb의 발굴 개발 파이프라인으로 이어졌는지 알아보세요.
Over the past three years, the development of treatments for COVID-19 gained substantial momentum. Vaccination has been at the forefront of the battle. Through mRNA administration, vaccines prompt your cells to produce a harmless version of the spike protein, thereby stimulating your immune system to produce antibodies to fight off the potential threat. The question is, what happens when you have already contracted COVID-19, or your immune system is compromised such that you are at high risk of hospitalization even after vaccination. That is where monoclonal antibodies step in by targeting and neutralizing the virus after it invades the body.
Featured podcast
On the newest podcast with Drug Target Review (Episode 6 - mAbs and SARS-CoV-2 with Dr. Carter Mitchell & Dr. Sharath Madasu, Kemp Proteins), Carter Mitchell, Chief Scientific Officer and Sharath Madasu, Manager of Protein Characterization of Kemp Proteins, discussed the role of monoclonal antibodies (mAbs) against COVID-19 and how the pandemic has shaped their discovery and development.
Drug Target Review · Episode 6 - mAbs and SARS-CoV-2 with Dr Carter Mitchell & Dr Sharath Madasu, Kemp Proteins
Table of contents
Why are mAbs effective against SARS-CoV-2?
Monoclonal antibodies (mAbs) act on viruses through viral neutralization. They are effective because they interrupt the process by which the virus either recognizes the host or the virus is internalized.
Working mechanism of monoclonal antibodies
In SARS-CoV-2, the aim is to disrupt the binding of the spike protein with ACE2 receptors, prohibiting the entry of the virus into the host cells. The spike protein mediates the binding through its recognition binding domain (RBD). Currently, most of the neutralizing monoclonal antibodies are raised against the RBD.
How effective are monoclonal antibodies against COVID-19 variants?
When it comes to effectiveness, it is difficult to give a single answer because of the growing number of variants. Efficacy varies across the variants. For instance, although most Emergency Use Authorized antibodies work on the Alpha and the Delta Variants, they exhibit a lower efficacy in the Omicron Variant.
The challenge with Omicron is that there are at least 36 mutations on the spike protein, some of which are located on the RBD. These mutations result in differential glycosylation, allowing the virus to evade previously-formed immunologic responses or monoclonal antibody neutralization.
Fortunately, newer antibodies, such as Sotrovimab, managed to retain their neutralizing activity in the recent variants, so there is still a glimmer of hope. Nevertheless, efficacy may highly vary. Some antibodies are very effective against Alpha and Beta Variants but not against the Gamma or Delta variants. In contrast, the antibodies effective against Delta and Alpha are much less effective against the Beta, Gamma and Omicron variants.
The impact of a particular variant mutation on EC50 depends on whether the mutations are at the binding epitope or not.
Advantages of mAbs over other Covid-19 therapies
The level of certainty in the required dosage is one of the main advantages of monoclonal antibodies in COVID-19 treatment.
According to Dr. Madasu, the success rate of convalescent plasma therapy relies heavily on the donor. Madasu further explains: “With the convalescent plasma therapy, A) you are expecting that the donor is still producing neutralizing antibodies, and B) Donor is producing sufficient amounts to be effective at all. With monoclonal antibodies, we know exactly how much of a neutralizing antibody we are administering to the patient, which is a huge advantage.”
Convalescent plasma therapy also brings about a substantial risk of plasma incompatibilities between patients. The use of monoclonal antibodies as an effective COVID-19 treatment method reduces these risks significantly.
Dr. Mitchell emphasizes another noteworthy feature of mAb therapy, the ability to produce a subset of pseudo polyclonal mixture that can neutralize any sort of variant that might emerge in the future. One might ask: How could we be so certain that these antibodies will be effective against future variants? Since each subset has a specific binding epitope, the mutations can quickly be recognized by one or a combination of mAb subsets.
Dr. Mitchell gives a plausible example of the potential benefits of polyclonal mixtures. “Five years from now, if a new type of variant comes out, we might be able to use a monoclonal antibody raised against the wild type PLUS one that came out in 2023 as a combination therapy to have more efficacy against that particular variant.”
The effect of a sudden pandemic
Monoclonal antibody development has undeniably been moving forward. The question remains: would the advancements in monoclonal antibody production still have taken place had it not been for the COVID-19 pandemic?
Although the process for monoclonal antibody development had already been established, the pandemic created a sense of urgency. Dr. Madasu also believes that people have an easier/better understanding of antibody therapies than that of vaccines. During the early phase of the pandemic, it was not clear how effective a vaccine would be, so certain sections of people may have hesitated to take the vaccine. On the other hand, people had a higher sense of understanding for antibodies, so antibody-based therapies were more widely-accepted.
The pandemic not only accelerated the improvement of existing strategies but also prompted the development of novel techniques. Technologies, such as high throughput surface plasmon resonance (SPR), high throughput biolayer interferometry (BLI), have garnered interest during the pandemic. In addition, high throughput dynamic light-scattering (DLS) and FLD have also improved a lot. One of the newer technologies was the artificial intelligence-based De Novo monoclonal antibody design, which increased the speed and complexity of antibody discovery platforms.
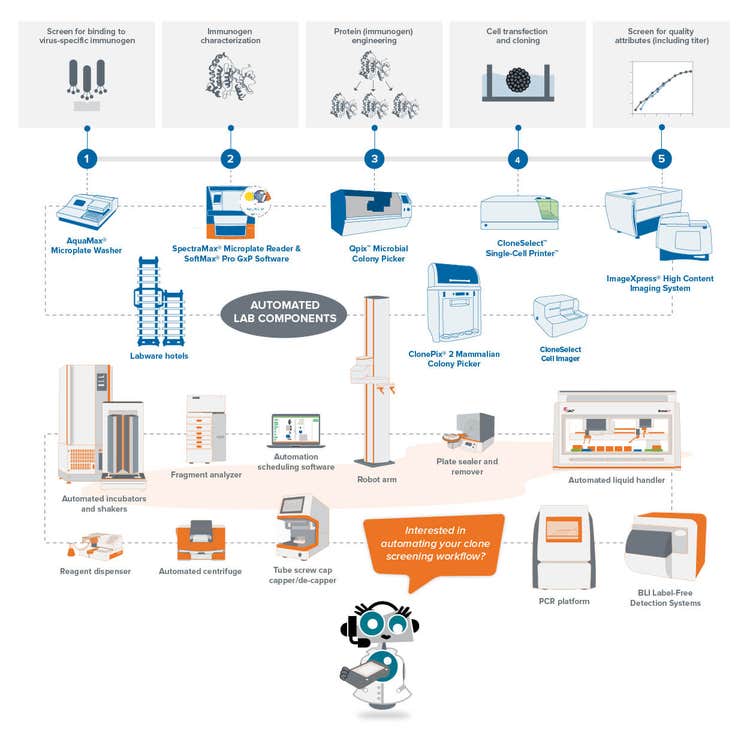
COVID-19 monoclonal antibody workflow
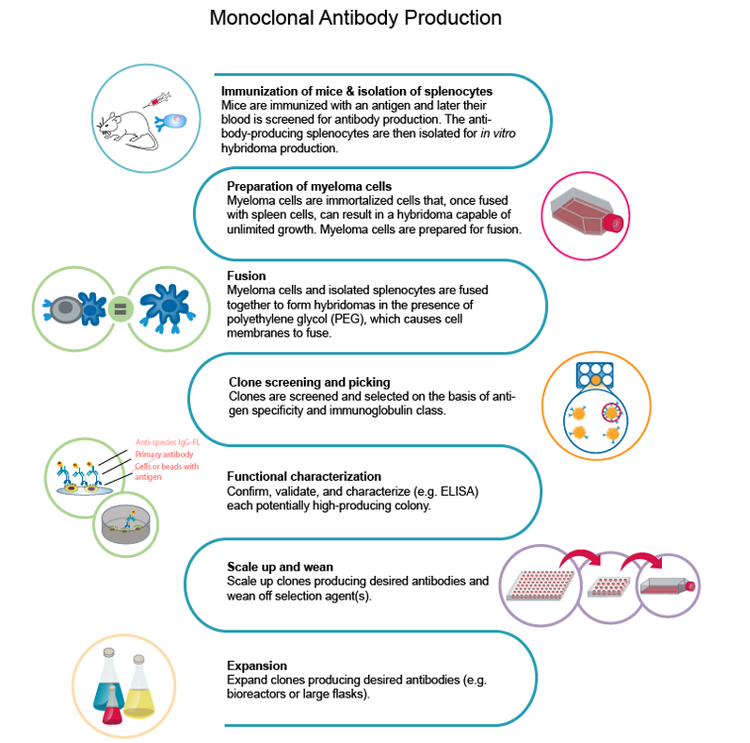
The first step to constructing the workflow is choosing the antigen to use for developing monoclonal antibodies. In the initial stage, immunization occurs by administering the antigen into an animal that develops an immune response. Then, the beta cells generated during the animal immune response are isolated, followed by fusion with a myeloma cell to generate the hybridoma.
According to Dr. Mitchell, the key to a successful initial stage is the optimization of the antigen. “In the case of COVID-19, antibodies are mainly raised against the spike protein, which is a trimer. The spike protein has a propensity of forming high molecular weight aggregates, which may not be great for immunization strategy”.
To add to that, glycosylation patterns of the spike protein highly vary across cell types, let alone different species.
That’s why one has to be very careful about antigen selection. Even a slight deviation of a single antigen glycan (e.g., in different variants) can significantly impact the success rate of the antibody.
https://main--moleculardevices--hlxsites.hlx.page/applications/monoclonal-antibody-production
Challenges and bottlenecks
Immunization
One of the main challenges is the comprehension of the viral genome. Research teams usually had to synthesize and purify antigens and optimize their expression. However, to evaluate the success of expression and purification, they had to rely on the collaborative nature of scientists to publish the information from their own labs. Overall, the immunization of an animal and the generation of hybridoma alone could take up to eight weeks.
단일 세포 분리
Upon identifying the best quality antigen for the immunization strategy, the next challenge is to get a sufficient number of B cells for the single-cell isolation of hybridoma.
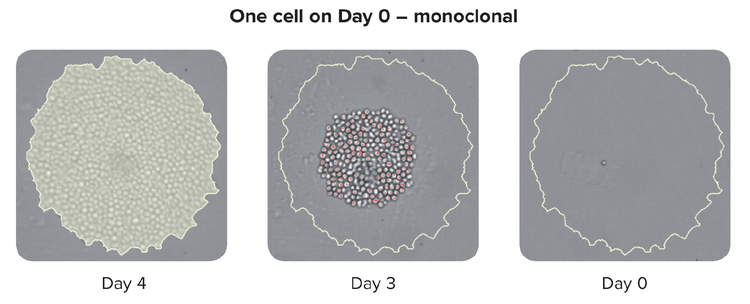
The traditional method to grow these hybridomas is a semi-solid medium that allows the single cell to form a colony. This used to be a slow process but has since gained momentum with robotic instruments that allow printing single cells into each well of a microwell plate, thereby improving both the throughput and efficiency.
It is important to note that advanced single-cell isolation tools also provide increased clonal outgrowth efficiency and monoclonality verification that the colony was generated from a single cell.
Large-scale monoclonality
For large-scale monoclonality, one needs to generate the recombinant form of a single-cell-isolated hybridoma because single-cell isolation is insufficient for clinically-relevant mAb generation. The approval for clinical trials would require the recombinant generation of the mAb and its formulation into a human antibody that allows for the appropriate immunologic response.
To form stable cell lines to generate mAbs at sufficient concentrations, you need to sequence and manipulate the gene into a human construct. CHO cell lines are used for the recombinant generation, with the aim of 8-20 g/L mAb production.
https://main--moleculardevices--hlxsites.hlx.page/applications/monoclonality
Time and financial constraints
Time and cost are key factors to consider in mAb workflows
Although the generation of stable clones can be achieved in 12 days upon initial feeding, full monoclonal assurance could take up to six months with traditional cloning methods. More importantly, generating a fully-realized mAb structure can be expensive. The solution would be to form single-chain variable fragment antibodies or VHH nanobodies. These are the smallest antibody fragments possible exhibiting specific binding affinity for an antigen. Dr. Mitchell summarizes why this approach is so powerful:
“By converting mAbs into smaller nanobodies, we can produce them in E. coli in a much more cost-effective manner. That drives down the costs, making it a broadly applicable therapeutic as opposed to those only accessible in developed countries.”
According to Dr. Madasu, their stable structures make distribution into less-developed countries more effortless. “We need to think about other lesser developed countries without access to storage facilities. Some of these nanobodies are pretty stable and they could be stored at less stringent conditions.”
Clearly, antibody-to-nanobody conversion is a crucial step to distribute mAbs-based therapies across the globe at a lower cost. Especially in less-developed countries with insufficient storage facilities, nanobodies would still remain stable in extreme temperatures.
Ensuring monoclonality and accelerating COVID-19 mAb production with automation
Monoclonality assurance
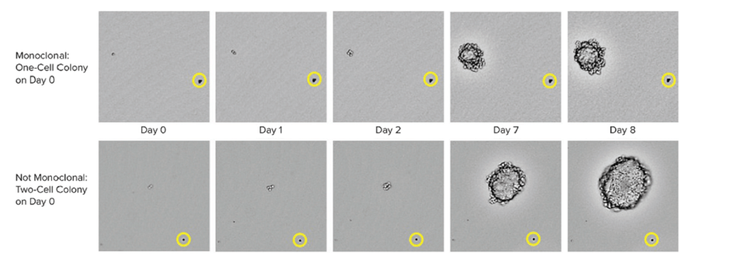
Methods to ensure monoclonality at scale include automated technologies like single-cell printing or colony-picking. When picking an isolated colony from the HAT medium, the instrument takes images over a time-lapse from day zero after picking the colony. The eye-witness proof constitutes evidence of monoclonality. To assure high-performing clones labs can use cell-based or immune-based assays combined with image-based methods. This combined strategy ensures that you have monoclonality and that your clones are secreting a single variety of the antibody. As a final step in the process, it is also helpful to run epitope binding assays to determine if the epitope binding is uniform.
https://main--moleculardevices--hlxsites.hlx.page/applications/monoclonality
The role of robotics in turnaround time
The ultimate way to decrease the development timeline is to implement high throughput robotics to interrogate the clones. In automated mAb workflows for COVID-19, automated purification has been of immense help to understand the behavior of the clones and the possibility of the monoclonal antibody making high molecular weight aggregates.
Utilizing robotics in a high throughput manner is always an advantage in bringing novel therapeutics to market, This method provides a better overview of the protein space or epitope space. Having a high number of clones maximizes your chances of obtaining mAbs that cover all the desired attributes instead of having a single mAb with partial coverage of the antigen.
With high-throughput workflows, one can narrow focus into a subset of mono-clones with the highest yield. You can then move forward with the highest-ranked subsets to run further assessments in animal studies and toxicology tests. You would also save time by eliminating subsets not suitable for large-scale purifications, which prevents monetary drain during the laboratory’s COVID-19 research journey.
Automation of mAb lab processes in the future
Many COVID-19 research laboratories have already implemented automated processes since they are able to generate data for thousands of clones a week. Comprehension of mAb therapeutic mechanisms is much more likely with automated synthesis, expression, purification, and biophysical characterization.
More importantly, automation paves the way for unbiased analysis methods and selection criteria. Objective analysis is necessary for diversifying the library of antibodies for a broad range of variants and mutations to avoid scaling up antibodies that only treat a narrow set of variants.
Future of SARS-CoV-2 research and the role of monoclonal antibodies
mAb-based therapeutics have been on the United States market since 1986 with the first mAb FDA approval of muromonab-CD3 (Orthoclone OKT3) development to reduce acute rejection in patients with organ transplants. (1) Current COVID-19 research increases the significance of monoclonal antibodies in diagnostics as well. In fact, monoclonal antibodies played a vital role in detecting SARS-CoV-2 variants.
Besides diagnostics, current mAbs help researchers determine necessary modifications and even inform the development of vaccine candidates. In investigating whether a reference mAb can neutralize a virus variant, researchers can decide whether to develop more efficient novel therapeutics.
While mRNA-based vaccines have been at the center of the battle against COVID-19, monoclonal antibodies have been behind the scenes of various applications, from protein-based vaccines to antibody-based therapeutics.
Dr. Madasu and Dr. Mitchell believe that going forward, monoclonal antibodies could be applied in particular to the unvaccinated population, including infants, patients with comorbidities (who do not respond well to vaccines), and people hesitating to get vaccinated. As mentioned earlier, the key is to produce cost-effective mAb-based therapeutics and make them as widely available as possible, by increasing efficiency in the discovery and production process.
Monoclonal antibody discovery for COVID-19 with Molecular Devices’ lab automation solutions
Clone screening is one of the bottlenecks of monoclonal antibody discovery because you have to test and analyze thousands of cells with respect to the target antigen. Automated clone screening workflows can help overcome this burden by reducing hands-on time and unifying and standardizing data extracted from multiple processes.
Molecular Devices has constructed integrated workflow solutions for the essential steps. Automated cell-line development workflows aim to produce monoclonal cell lines producing consistent and sufficient levels of the target therapeutic protein. Automated molecular cloning workflow seeks to minimize errors and contamination during the isolation of DNA sequences, which are propagated as vectors into the species of your choice.
These workflows maximize the yield of target proteins while providing an opportunity to integrate other instruments for a fully automated workcell with robotics. . Overall, such workflows are cost-effective, time-saving, and easy to modify with changing research goals.
On our Lab Automation for High-Throughput Clone Screening page, you can learn more about cell line development workflows in greater depth. Don’t forget to check out our monoclonal antibody application page to view mAb production methods and the intricacies of every step involved.
- Wang, S. S., Yan, Y. S., & Ho, K. (2021). US FDA-approved therapeutic antibodies with high-concentration formulation: summaries and perspectives. Antibody therapeutics, 4(4), 262–272. https://doi.org/10.1093/abt/tbab027