
Application Note
Measure cell migration using a simple scratch assay with timelapse live-cell imaging
- Maintain live cells for multiple days inside the instrument during timelapse imaging
- Keep instrument free by using discontinuous timelapse and transporting plate between imager and incubator
- Use fluorescently stained or unlabeled cells
- Scale up to 96-well microplates for drug screening
Jayne Hesley | Imaging Applications Scientist | Molecular Devices
Introduction
The movement or migration of cells has long been studied to elucidate the physiological mechanisms of angiogenesis, embryogenesis, cancer metastasis, immune responses, and wound healing. This application describes a simple, medium to high throughput wound healing assay for measuring the closure of a man-made “wound” in a live cell monolayer. By conducting the assay in a 96-well microplate, several compounds, with adequate replicates, may be tested in one controlled experiment. In this assay, cell proliferation, migration, and spreading all contributed to the cells’ ability to close the wound. Automated timelapse imaging using transmitted light or live cell-compatible fluorescence provided the means to measure the progress of the “healing”. The images from every time point may be objectively analyzed with high-content image analysis software so that the data can be plotted as a timecourse or evaluated individually at specific time points.
Methods
In the following experiments, cells were plated into 96-well polystyrene imaging plates at a concentration optimized to reach confluency after growing overnight in an incubator. A cell density of 40,000 cells/well was used for two cell lines: HT1080 human fibrosarcoma and U2OS human osteosarcoma. After cells were allowed to adhere overnight, twelve 20 µL disposable pipet tips were loaded onto a manual multi-channel pipettor and dragged firmly across each row of wells from top to bottom to simultaneously create a thin scratch in the surface of each monolayer. The plate was inspected under a light microscope to confirm uniform scratching, then the media was aspirated, and wells were rinsed one time with 200 µL warm DPBS before the addition of 200 µL of compounds prepared in media. The HT1080 cell line was labeled at the time of compound treatment by the addition of 100 nM siRactin (Spirochrome). The U2OS cell line was already stably transfected to express RFP-actin. The microplate was placed into the ImageXpress Micro 4 system equipped with environmental control and transmitted light options. The plate was automatically imaged using the 4X objective to acquire one field of view/well with transmitted light, RFP, and siR-actin compatible fluorescent wavelengths every hour for 20 hours and then again at 22 hours. After timelapse imaging, the plate was fixed using 4% formaldehyde. An alternative protocol can be used where the microplate is imaged, returned to the incubator until the next time point, and then reimaged to append to the previous read(s), thus constructing a “discontinuous” timelapse.
Transmitted light: 1-5 ms exposure
Cy5/siR-DNA or siR-Actin: 400-500 ms
TRITC/Actin-RFP: 200-400 ms
Transmitted Light, Cell Type B
Cell Scoring – Cy5 or TRITC nuclei and cytoplasm
The timelapse images were analyzed with MetaXpress® software either with a custom module utilizing a preconfigured transmitted light segmentation algorithm or with a module utilizing fluorescence segmentation for the actin staining of the cells.
Use transmitted light to measure open area of the scratch
A 4X objective allows a large area of the well to be imaged in a 96-well plate using just one field-of-view if the scratches are carefully made close to the center (Figure 1). To save the most time, a quick pre-read of all the wells in the plate may be conducted and then only wells containing passable scratches can be imaged during the timelapse experiment.
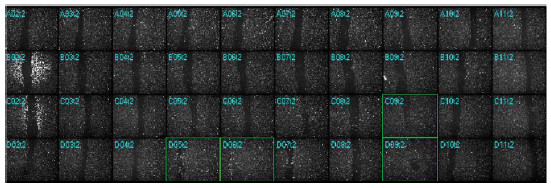
Figure 1. At Time 0 after scratching, a montage of the plate thumbnails allows an evaluation of efficiency and uniformity of the wounding between the wells. Wells outlined in green show incomplete or missed scratches and were omitted from analysis.
Transmitted light images are suitable for segmentation in MetaXpress software, either by using a simple transmitted light module to identify cells and calculate the area of the well covered by cells or, for images with low contrast or numerous artifacts, by adding some image processing and filtering steps from the Custom Module Editor toolbox to enable accurate measurement of the open wound area (Figure 2). Although a fluorescent image was also acquired for each cell type and the analysis results using transmitted light images in these experiments were satisfactory, the fluorescence data is not shown.
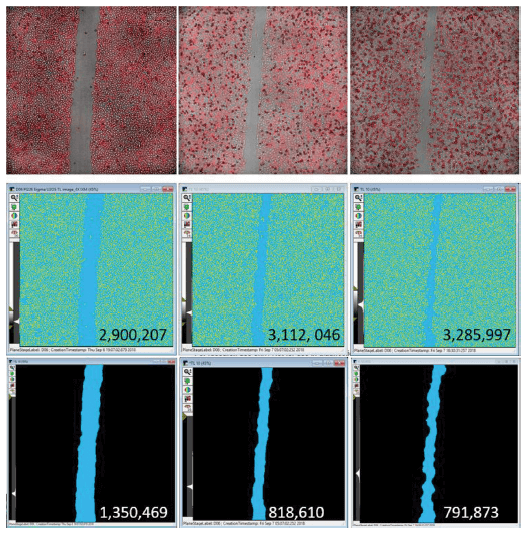
Figure 2. A. Top images show an overlay of adherent U2OS cells acquired in both transmitted light and TRITC (for Actin-RFP) with a 4X objective at three representative timepoints: 1 hour, 11 hours, and 23 hours post-treatment with 100 nM Colchicine. B. The middle panels show the mask generated using Transmitted Light (Cell B) Segmentation module in MetaXpress software. The yellow areas were identified as cells and the total area under the yellow mask is reported. C. The bottom panels show the light blue mask generated using a custom module to identify the area that is free of cells. The calculated result is super-imposed upon the mask image showing the decrease in scratch area overtime.
The averaged data from each treatment may be plotted against time to show the progression of the cell migration into the scratch area (Figures 3 and 4). Any well may also be easily converted into a movie using a single wavelength or an overlay of any wavelengths acquired.
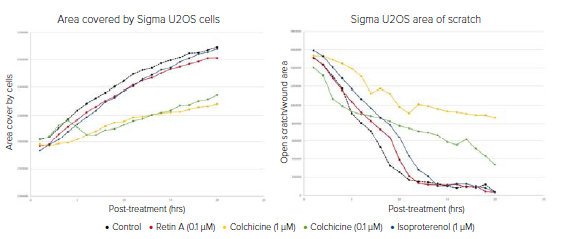
Figure 3. Data plotted over 20 hours to show the progression of control and treated U2OS cells migrating into the scratch area. Left graph shows results from a simple calculation of total well area covered by cells as determined by the transmitted light images. Total cell area increases over time at varying rates depending on the effect of compound treatments on cell migration. Right graph shows the closure of the wound as measured by decreasing wound area. The graphs correlate to the example segmentation images seen in Figure 2. With both analyses, it can be concluded that colchicine significantly inhibits cell migration.
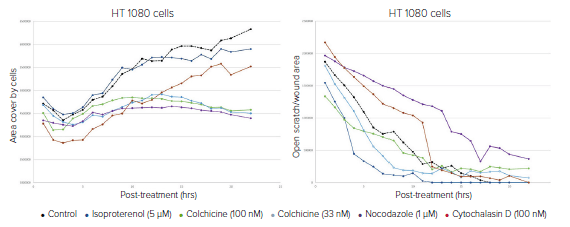
Figure 4. Data plotted over 20 hours to show the progression of control and treated HT1080 cells migrating into the scratch. Left graph shows results from a simple calculation of total well area covered by cells as determined by the transmitted light images. Total cell area increases over time at varying rates depending on the effect of compound treatments on cell migration. Right graph shows the closure of the wound as measured by decreasing wound area. With both analyses, it can be concluded that colchicine significantly inhibits cell migration.
Get valid results using different cell types
By using the Transmitted Light Analysis Module for Cell Type B (which is optimized for larger cells), results were generated for both HT1080 and U2OS cells. The timelapse data for both of the cell types correlated with a visual evaluation of the images.
Save time and storage space by acquiring only key timepoints
In a multi-user environment, a long timelapse experiment can monopolize the imaging instrument, preventing other scientists from running their assays. With the discontinuous timelapse feature of Metaxpress software, version 6.5, plates of live cells may be retrieved from an incubator and imaged whenever desired and the individual acquisitions will be appended into one experiment for seamless timelapse analysis (Figure 5). This will also eliminate the generation of too much extraneous data when the cells may take days to fully migrate into the scratch. It also saves space in the database which can become a premium when running timelapse assays in multi-well microplates.
Figure 5. Discontinuous timelapse acquisition is accomplished by entering a barcode. When enabled, all timepoints are appended to the same file containing the barcode and may be analyzed together as if they were collected as a standard timelapse experiment.
Analysis of 20 or more timepoints across 96 wells can take a significant amount of time to complete but, by running the analysis with MetaXpress PowerCore, which utilizes parallel processing across multiple computer cores, the analysis can be completed up to 10 times faster.
Conclusion
The ImageXpress Micro system allows researchers to quantitate, over time, cell migration into a cell-free area created by “wounding” a confluent monolayer. Timelapse images may be acquired automatically at defined intervals or manually by moving the plate in and out of the instrument, for example if the instrument does not sport the environmental control option needed to maintain the live cells or if other lab users need to acquire images on the same instrument during a long timelapse experiment. The instrument can accommodate scratch assays performed in low density (12-48 well) tissue culture plates or in 96-well microplates using low magnification objectives from 2X-10X and the software can stitch sites together if more than one field of view is necessary. The integrated MetaXpress software offers analysis for transmitted light or fluorescence images to generate data ranging from a simple area covered by cells to more complex measurements such as width of the wound or area of the scratch.
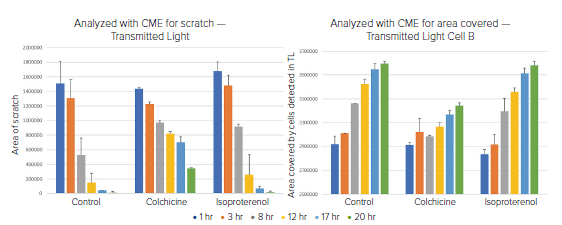
Figure 6. Six timepoints were acquired at varying intervals and analyzed for A) Area of the open wound or B) Area of the well covered by cells. Both analyses were done using a transmitted light image. In both cases, it is clear that colchicine slowed the migration of the cells into the wound.