
Application Note
Measuring cell health on the SpectraMax iD3 reader with cell viability assays
- Fully optimized assays for SpectraMax iD3 reader save time on assay development
- Simple, homogeneous workflow minimizes cell handling
- High-resolution touchscreen allows for quick set up of methods
- Preconfigured protocols in SoftMax Pro Software expedite data analysis
Introduction
Measuring cell health from the multiple vantage points of viability and apoptotic pathways can provide insight into the impacts of a variety of experimental treatments including drug candidates, pathway activators and inhibitors, and reporter genes.
One of the most popular methods for assaying cell health is fluorescence detection with a microplate reader. In this application note, we report the use of the EarlyTox™ Cell Viability suite of assay kits on the SpectraMax® iD3 Multi-Mode Microplate Reader (Figure 1).
Figure 1. Representative experimental workflow for a cell viability assay.
EarlyTox Live/Dead Assay Kit
The kit contains two markers for live or dead cells that are suitable for use with mammalian cells. Calcein AM is a widely used live-cell marker. The nonfluorescent calcein AM permeates the intact cell membrane and is converted into fluorescent calcein by intracellular esterases. Live cells are stained with intense green fluorescence in the cytosol. For cell proliferation assays, or other assays where live cell staining only is desired, calcein AM can be used as a standalone reagent as provided in the EarlyTox™ Live Cell Assay Kit (P/N R8342 for Explorer kit, P/N R8343 for Bulk kit).
Ethidium homodimer-III (EthD-III) is virtually non-fluorescent and impermeant to an intact plasma membrane. In the event of compromised cell membrane integrity that is associated with cell death, EthD-III enters cells and binds to nucleic acids, resulting in bright red fluorescence in dead cells. Cytotoxic events that affect cell membrane integrity can be accurately assessed using this method.
Fluorescent signals from calcein and EthDIII can be detected using the SpectraMax iD3 reader and rapidly analyzed using the EarlyTox Live Dead preconfigured protocol in SoftMax® Pro Software.
EarlyTox Glutathione Assay Kit
This kit uses monochlorobimane (MCB), a cell permeant dye with a high affinity for GSH, to detect cellular GSH levels. Reaction of the dye with GSH is catalyzed by endogenous glutathioneS-transferase (GST) enzymes and results in the generation of blue fluorescence with excitation at 394 nm and emission at 490 nm. The fluorescence intensity corresponds to the amount of GSH present in cells, which increases with apoptosis. Unlike representative competitor assays, the EarlyTox Glutathione assay can be used on live, intact cells in a microplate format without the need for cell harvest and centrifugation, lysis, or other timeconsuming manipulations that can lead to variability in results. Instrument settings and analysis in SoftMax Pro Software are simplified with the preconfigured EarlyTox Glutathione protocol.
EarlyTox Caspase-3/7 R110 Assay Kit
The EarlyTox Caspase-3/7 R110 Assay Kit provides a single-step, homogenous assay that is specifically designed for microplate readers. The fluorogenic substrate (Ac-DEVD)2-R110 contains two DEVD consensus target sequence and is completely hydrolyzed in cell lysate by the enzymes in two successive steps. Hydrolysis of both DEVD peptides releases the green fluorescent dye rhodamine 110 (R110), resulting in a substantial fluorescence increase, with excitation at 490 nm and emission at 520 nm. A simplified workflow reduces the number of cells typically required for such an assay and also the variability typically encountered with multiple steps. A preconfigured EarlyTox R110 acquisition and analysis protocol is included in the SoftMax Pro protocol library.
Materials
- EarlyTox™ Live/Dead Assay Kit
- Explorer Kit (2-plate size, Molecular Devices P/N R8340)
- Bulk Kit (10-plate size, Molecular Devices P/N R8341)
- EarlyTox™ Glutathione Assay Kit
- Explorer Kit (2-plate size, Molecular Devices P/N R8344)
- Bulk Kit (10-plate size, Molecular Devices P/N R8345)
- EarlyTox™ Caspase-3/7 R110 Assay Kit
- Explorer Kit (2-plate size, Molecular Devices P/N R8346)
- Bulk Kit (10-plate size, Molecular Devices P/N R8347)
- HeLa cells (ATCC P/N CCL-2)
- Staurosporine (Sigma P/N S5921)
- 96-well black, clear-bottom microplates (Corning P/N 3904)
- SpectraMax iD3 Multi-Mode Microplate Reader
Methods
Cell treatment
HeLa cells were plated at 20,000 cells per well in 100 µL per well in a black, clear-bottom microplate. They were allowed to attach and grow overnight in a 37°C, 5% CO2 incubator. The cells were then treated to induce apoptosis. For the Live/Dead assay, they were treated for 24 hours with 1:2 dilutions of staurosporine from 10 µM down to 40 nM. For the Glutathione and Caspase-3/7 R110 assays, they were treated for four hours with 1:2 dilutions of staurosporine from 5 µM down to 5 nM. Four replicates were run at each concentration.
EarlyTox Live/Dead Assay Kit
A 2X working solution of calcein AM/ EthD-III was prepared by adding calcein AM and EthD-III stock solutions to PBS for a concentration of 6 µM for each dye. 100 µL of the 2X working solution was added to each assay well, resulting in a final volume of 200 µL and a final concentration of 3 µM for each dye. The plate was incubated at room temperature for one hour. It was then read from the top and bottom on the SpectraMax iD3 reader using a preconfigured protocol in SoftMax Pro Software with the settings indicated in Table 1. Note: removal of medium, followed by addition of a 1X solution of calcein AM and EthD-III, is optional and can help reduce background fluorescence if necessary.
The preconfigured EarlyTox Live Dead assay protocol in SoftMax Pro Software automatically calculated green/red ratios, which were then plotted against compound concentration. The protocol enables calculation of percentage of live and dead cells in the experimental cell samples where such analysis is required. For these calculations it is necessary to set up additional controls in the assay plate.
EarlyTox Glutathione Assay Kit
A 20 µM MCB working solution was prepared by diluting 20 µL of 10 mM MCB stock solution in 10 mL of PBS. Medium was removed from the cells in the assay plate and replaced with 100 µL of MCB working solution. Cells were incubated at 37°C. At one hour and two hours after reagent addition, fluorescence intensity was measured on the SpectraMax iD3 reader using the settings indicated in Table 1.
Lm1: Ex = 495 nm, Em = 530 nm
Lm2: Ex = 530 nm, Em = 645 nm
PMT gain: Automatic
Integration time: 500 ms
Read from bottom
PMT gain: Automatic
Integration time: 500 ms
Read from bottom
PMT gain: Automatic
Integration time: 500 ms
Read from bottom
Table 1. Settings for SpectraMax iD3 Multi-Mode Microplate Reader. Similar settings will work for other SpectraMax readers with fluorescence detection.
EarlyTox Caspase-3/7 R110 Assay Kit
Substrate assay buffer was prepared by adding enzyme substrate (AC-DEVD)2-R110 (2 mM) to cell lysis/assay buffer at a ratio of 50 µL to 1 mL buffer. 100 µL of substrate assay buffer was added to each well, resulting in a final volume of 200 µL per well and a final concentration of 50 µM substrate. The samples were then incubated at room temperature. Fluorescence was measured on the SpectraMax iD3 reader at one hour and two hours after reagent addition using the settings indicated in Table 1.
Results
EarlyTox Live/Dead Assay Kit
HeLa cells treated with staurosporine showed a clear concentration response, which was easily quantified as the ratio of live (green fluorescence) to dead (red fluorescence) signal (Figure 2). Overall, RFU values were higher for cells incubated for two hours than for cells incubated for one hour (data not shown). The EC50 value calculated from a 4-parameter curve fit of the data was 300 nM.
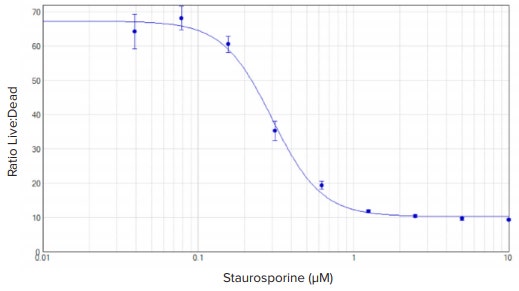
Figure 2. EarlyTox Live/Dead assay: concentration response curves for HeLa cells treated with staurosporine for 24 hours. Cells were incubated in calcein AM and EthD-III for one hour. Concentration curve was plotted using the ratio of green (530 nm emission) over red (645 nm emission) RFUs at the Y-axis. A 4-parameter curve fit was applied in SoftMax Pro Software. EC50 value was 300 nM.
EarlyTox Glutathione Assay Kit
HeLa cells treated with staurosporine for four hours exhibited an apoptosisassociated decrease in intracellular glutathione that was measured using the EarlyTox Glutathione assay. A decrease in fluorescence with increasing staurosporine concentration was detected with the SpectraMax iD3 reader. Results were graphed in SoftMax Pro Software using a 4-parameter curve fit. Fluorescence intensity values increased over time, but similar EC50 values of 263 nM and 295 nM were obtained for assay incubation times ranging from one to two hours (Figure 3).
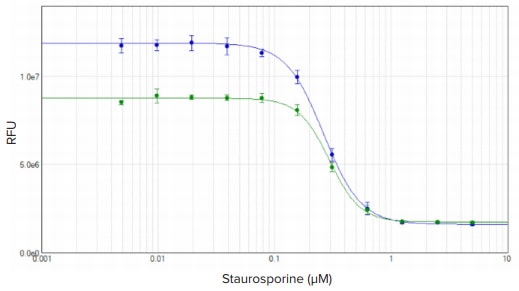
Figure 3. EarlyTox Glutathione assay: concentration response curves for HeLa cells treated with staurosporine for four hours. Cells were incubated in reagent for one (green plot) or two (blue plot) hours. Concentration curves were plotted using a 4-parameter curve fit in SoftMax Pro Software. EC50 values were 263 nM and 295 nM, respectively.
EarlyTox Caspase-3/7 R110 Assay Kit
HeLa cells treated with staurosporine for four hours exhibited an apoptotic response that was measured using the EarlyTox Caspase-3/7 R110 assay. Fluorescence corresponding to apoptotic cells was detected with the SpectraMax iD3 reader. Results were graphed in SoftMax Pro Software using a 4-parameter curve fit. Cells incubated in substrate for one or two hours had similar EC50 values of 193 nM and 200 nM (Figure 4).
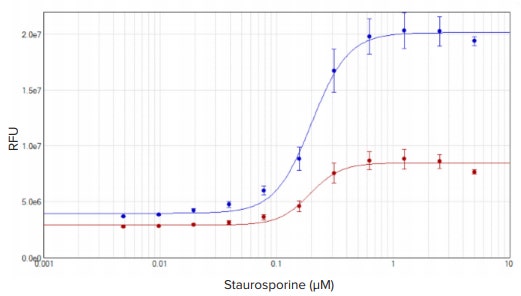
Figure 4. EarlyTox Caspase-3/7 R110 assay: concentration response curves for HeLa cells treated with staurosporine for four hours. Cells were incubated in reagent for one (red plot) or two (blue plot) hours. Concentration curves were plotted using a 4-parameter curve fit in SoftMax Pro Software. EC50 values were 193 nM and 200 nM, respectively.
Conclusion
Used together with the SpectraMax iD3 reader, the EarlyTox Cell Viability Assay Kits enable direct measurement of live and dead or apoptotic cells with a simple workflow and the increased throughput offered by a microplate format. Consistent results are obtained over time offering workflow flexibility. Preconfigured SoftMax Pro Software protocols provide optimized instrument settings and speed the time to results with automatic data analysis.
SpectraMax iD3 Multi-Mode Microplate Reader
The SpectraMax iD3 reader features a large, high-resolution touchscreen interface with an embedded software package allowing users to set up custom protocols, use pre-loaded protocols, and run experiments without the need for a dedicated computer workstation. Built-in near-field communication (NFC) functionality enables access to custom protocols and results with a single tap, saving precious time.
简介
从活性和凋亡等多个表征评估细胞健康状 态能协助我们深入了解多种情况下,包括 候选药物,特定通路的激活、抑制剂处理 以及报告基因的表达所带来的影响。目前 有多种方法可用于测定细胞健康状态,其中 比较常见的是通过微孔板读板机结合荧光检 测法。该应用指南展现了在SpectraMax® iD3 多功能微孔板读板机上应用EarlyToxTM 系 列细胞活性检测试剂盒进行细胞健康评估。

图1:细胞活性检测实验流程代表图
EarlyToxTM细胞死活检测试剂盒
该试剂盒包含两个适用于标记哺乳动物细 胞死活的标记物。其中,Calcein AM广 泛用于标记活细胞。非荧光的Calcein AM 可穿透完整的细胞膜,进入胞浆后在细胞 内脂酶的作用下转换成具有荧光的 Calcein。因此,在Calcein AM标记下活 细胞胞浆会发出强烈的绿色荧光。有些实 验中只涉及到活细胞的标记,如细胞增殖 实验等,仅需要EarlyToxTM Live Cell检测 试剂盒提供的Calcein AM(货号 R8342 for 探索包装,货号 R8343 大包装)。
Ethidium homodimer-III(EthD-III)是 几乎无荧光的标记物,其不具有穿透完整 细胞膜的能力。在细胞死亡过程中,往往 会出现细胞膜完整性的受损。此时, EthD-III可进入细胞并结合核酸,在死细 胞中产生强烈的红色荧光。EthD-III染色 可用于准确评估细胞毒性事件对膜完整性 的影响。
Calcein 和 EthDIII的荧光信号均可用 SpectraMax iD3微孔板读板机进行检 测,并能在SoftMax® Pro软件中结合预设 的EarlyTox Live/Dead 模板迅速完成分 析。
EarlyTox Glutathione 检测试剂盒
该试剂盒利用对GSH具有高亲和力的细胞膜 可渗透染料monochlorobimane(MCB) 进行细胞内GSH水平的检测。在内源 GlutathioneS-transferase(GST)酶处 理下,MCB与GSH进行反应并产生394 nm 激发,490 nm 发射的蓝色荧光,强度 与细胞内GSH的水平对应,并随着凋亡的 发生下降。与主流的检测方法不同, EarlyTox Glutathione检测法可直接在微 孔板中分析完整的活细胞,无需进行细胞 收集,离心等耗时且易引入误差的操作。 同时,预设的EarlyTox Glutathione模板 简化了仪器的设置和软件分析过程。
EarlyTox Caspase-3/7 R110 检测试 剂盒
EarlyTox Caspase-3/7 R110 检测试剂盒 专门为微孔板读板机提供了一步式均相检 测方法。荧光底物(Ac-DEVD)2-R110含 有2个DEVD 共识目标序列,并在细胞裂解 产物中相关酶的作用下逐步完全水解,释 放绿色荧光染料rhodamine 110(R110) 并伴随显著的荧光信号(490 nm激发, 520 nm发射)增加。简化的操作流程降低 了对细胞数量的需求,同时避免多步操作 所带来的误差。相应的SoftMax Pro模板 库中提供预设模板用于EarlyTox R110采 集和分析。
材料
- EarlyTox™ 细胞死活检测试剂盒
- 探索包装 (两块微孔板通量, Molecular Devices 货号 R8340)
- 大包装 (十块微孔板通量, Molecular Devices 货号 R8341)
- EarlyTox™ Glutathione 检测试剂盒
- 探索包装 (两块微孔板通量, Molecular Devices 货号 R8344)
- 大包装(十块微孔板通量, Molecular Devices 货号 R8345)
- EarlyToxTM Caspase-3/7 R110 Assay Kit
- 探索包装 (两块微孔板通量, Molecular Devices 货号 R8346)
- 大包装(十块微孔板通量, Molecular Devices 货号 R8347)
- HeLa 细胞 (ATCC 货号 CCL-2)
- Staurosporine (Sigma 货号 S5921)
- 96孔黑色底部透明微孔板 (Corning 货号 3904)
- SpectraMax iD3 多功能微孔板读板机
方法
细胞处理:
Hela细胞以100 µL体系,20,000个每孔的 密度铺于黑色底部透明板中。在37°C, 5% CO2 培养箱培养过夜,细胞贴壁后,用不 同的方法诱导凋亡。对于细胞死活检测, 用2倍梯度稀释浓度范围为10 µM 到40 nM的Staurosporine处理细胞24小时。对 于Glutathione 和 Caspase-3/7 R110 检 测,则用2倍梯度稀释浓度范围为5 µM 到 5 nM的Staurosporine处理细胞4小时。每 个药物浓度设置4重复。
EarlyTox 细胞死活检测试剂盒
配置2倍工作处理液:在PBS缓冲液中加 入Calcein AM和EthD-III 储液至染料终浓 度均为6 µM。每实验孔中加入100 µL 2倍 工作处理液使最终反应体系为200 µL,染 料终浓度均为 3 µM。室温孵育 1 小时后 用SpectraMax iD3微孔板读板机进行顶 部或底部读取。参数设置依照表1所示使 用SoftMax Pro 完成。注意,必要时也可 以先移除培养液后加入1倍工作处理液进 行染色,有利于降低背景荧光信号。
SoftMax Pro软件预设的EarlyTox Live Dead assay 模板会自动计算绿色荧光/红 色荧光比例,并根据药物浓度进行作图。 如有需求,模板也可以计算死活细胞的比 例,不过必须在实验板中设置额外的对 照。
EarlyTox Glutathione 检测试剂盒
配置20 µM MCB工作处理液:稀释 20 µL 10 mM MCB储液于10 mL PBS中。移除实 验板的培养基后每孔细胞加入100 µL MCB工作处理液,37°C进行孵育。孵育1 小时和2小时后用SpectraMax iD3微孔板 读板机按照表1的参数进行荧光检测。
Lm1: Ex = 495 nm, Em = 530 nm
Lm2: Ex = 530 nm, Em = 645 nm
PMT gain: Automatic
Integration time: 500 ms
Read from bottom
PMT gain: Automatic
Integration time: 500 ms
Read from bottom
PMT gain: Automatic
Integration time: 500 ms
Read from bottom
表1:SpectraMax iD3 多功能微孔板读板机参数设定。 类似的设定可用于其他具有荧光检测功能的SpectraMax微孔板读板机
EarlyTox Caspase-3/7 R110 检测试 剂盒
配置荧光底物工作液: 按照50 µL 每 1 mL 的比例加入酶底物(AC-DEVD)2-R110 (2 mM) 至细胞裂解或检测缓冲液中。 每孔细胞中加入100 µL荧光底物工作液, 使终体系为200 µL每孔,底物终浓度为 50 µ M。室温孵育1小时和2小时后用 SpectraMax iD3微孔板读板机按照表1的 参数进行荧光检测。
Results
结果 EarlyTox 细胞死活检测试剂盒
Staurosporine 处理在Hela 细胞上引起明 显的浓度反应关系,可以通过计算活细胞 (绿色荧光)和死细胞(红色荧光)的比 例轻易进行量化(图2)。一般来说,孵 育2小时的细胞样本的RFU高于孵育1小时 (结果未显示)。此次检测中使用4参数 曲线拟合出EC50值为300 nM。

图2:EarlyTox细胞死活检测: Staurosporine处理Hela细胞24小时的浓度反应曲线。 Calcein AM 和 EthD-III孵育细胞一小时后进行检测。依据绿(530 nm 发射波长)/ 红(645 nm发射波长)RFU 值的比值绘制浓度曲线。数据使用4参数曲线拟合。EC50 值为300 nM。
EarlyTox Glutathione 检测试剂盒
Staurosporine 处理4小时后HeLa细胞出 现凋亡,并伴随细胞内Glutathione水平 的下降,其可用EarlyTox Glutathione 检测 法进行分析。随着Staurosporine浓度的提 升,荧光信号逐渐下降并可用SpectraMax iD3 微孔板读板机检测。结果在SoftMax Pro 软件中使用4参数曲线拟合。虽然荧光信 号值随着时间的推移而增加,孵育1小时 和孵育2小时所得的EC5 0值是相近的263 nM 和295 nM (图3)。

图3:EarlyTox Glutathione 检测: Staurosporine处理Hela细胞4小时的浓度反应曲线。 细胞分 别和染料孵育1小时(绿线)和2小时(蓝线)。在SoftMax Pro 软件中使用4参数曲线拟合。 EC50 值分别为263 nM和295 nM。
EarlyTox Caspase-3/7 R110 检测试 剂盒
Staurosporine 处理4小时后HeLa细胞出 现凋亡并用EarlyTox Caspase-3/7 R110 检 测法进行分析。凋亡细胞对应的荧光信号 可用SpectraMax iD3微孔板读板机进行 检测。结果在SoftMax Pro软件中使用4参 数曲线拟合。底物孵育1小时和2小时所得 的EC50值是相近的193 nM 和200 nM (图4)。

图4. EarlyTox Caspase-3/7 R110 检测: Staurosporine处理Hela细胞4小时的浓度反应曲线。 细 胞分别和染料孵育1小时(红线)和2小时(蓝线)。在SoftMax Pro 软件中使用4参数曲线拟合。 EC50 值分别为193 nM和200 nM。
总结
结合SpectraMax iD3微孔板读板机, EarlyTox细胞活性系列试剂盒提供了直接, 方便的细胞死活或凋亡的检测方法,并且 使用微孔板进一步提升通量。不同孵育时 间的结果一致性增加了实验流程的灵活性。 SoftMax Pro 软件中预设的模板提供了优 化的检测参数设定并通过自动数据分析减 少获得数据所需的时间。
SpectraMax iD3 多功能微孔板 读板机
SpectraMax iD3微孔板读板机设计上引 入了大尺寸,高分辨率触摸屏并内嵌软件包 便于用户自定义模板,使用预设模板和在无 需专用电脑工作站的前提下进行实验。仅需 轻轻一点,内置的近场通讯(NFC)功能 就能提供自定义模板和数据,节约您的宝 贵时间。